Extraction and Drying of an Aqueous Solution Group Members. By May 27 2021.
Schematically Presentation Of Extraction Process For Precious Metals Download Scientific Diagram
A drying column consists of a pipet plugged with a small wad of cotton.
. It is then allowed to remain undisturbed for some time. CourseOrganic Chemistry CHM 207 Uploaded by. Solvent Extraction Methods.
To ensure all water is removed the organic solutions should be dried. Set up a simple distillation apparatus with a 100 mL rb. Boiling range of diethyl ether 0 C to 34C Boiling point of toluene 110C.
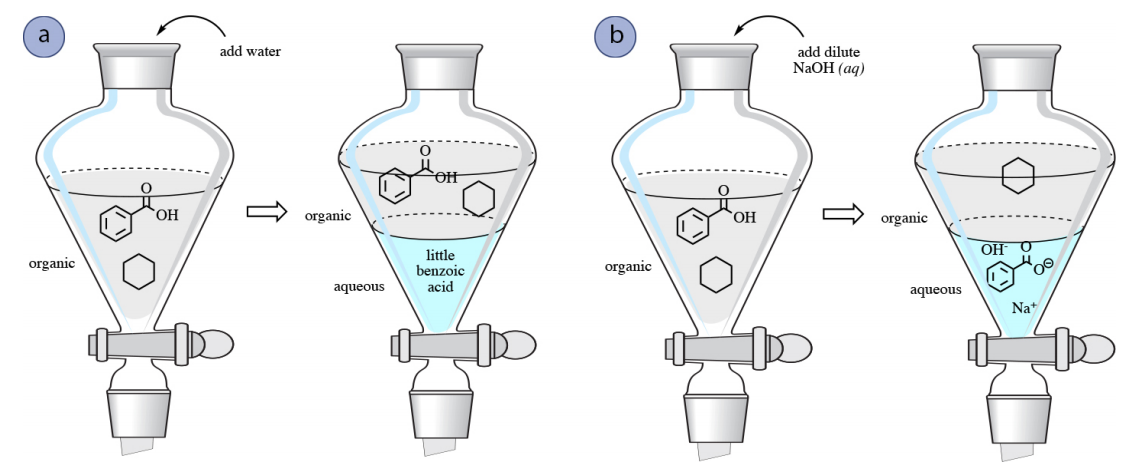
The aqueous solution of the given solute is taken in a separating funnel. To learn the techniques of separating toluene from water and other inorganic compounds by extraction. Explain why vacuum filtration is the method of choice for separating the benzoic acid from the neutralized aqueous solution.
Where a solid or liquid suspended or dissolved in one solvent is extracted into another. If you are uncertain check with the instructor. Drying involves letting an organic solution stand over a drying agent usually an inorganic salt.
50-100 ml of dichloromethane. 2 The handbook covers both vacuum and gravity filtration techniques. Nurul Fadhlin binti Shamsuri- ID 2021200634 Nur Ikram Hakim bin Huzaili- ID 2021801026 Lecturers Name.
Extraction and Drying of an Aqueous Solution Students name. Drying the Organic Solvent. To dry your organic product by this method place the organic solution in a separatory funnel.
To learn the techniques of separating toluene from water and other inorganic. Leave to stand for 2 minutes or until the solution form two stable layers. To learn the techniques of separating toluene from water and other inorganic compounds by extraction.
Add an amount of saturated aqueous sodium chloride less than or equal to the amount of organic solution you have. A drying agent such as magnesium sulfate can be used to further extract aqueous solvent from the organic solvent after extraction. These techniques were learned and studied thoroughly through the given YouTube links.
The possible mechanisms of non-destructive ion extraction are discussed. To learn the technique of separating toluene from water and other inorganic. Water and organic solvent will form separate layers and the solid or liquid.
There is a net transfer of one or more species from one liquid into another liquid phase generally from aqueous to. Reextract the organic layer from above two times with 30-ml portions of 1 M sodium hydroxide solution and combine these two aqueous extracts in another labeled flask. Dr Sharizal Hasan Date of the experiment.
The tap was opened and then drain the diethyl ether layer containing toluene into a dry conical flask. DISCUSSION Extraction is a fundamental technique that can be used to isolate one compound from a mixture. Drying of Aqueous Solution 25 Liters spraying vessel Drying of water base solutions to micron-sized particles.
Common examples of extraction are liquid-liquid extraction and the solid phase. Carbonyl Compounds - Reactions of Aldehydes and Ketones. It is mixed with the desired organic solvent.
- To learn the techniques of drying a dehydrated solution. Extract this solution two times with 30-ml portions of 1 M hydrochloric acid. The funnel is closed and its contents are shaken vigorously.
Add drying agent until the organic solvent is sufficiently dried of aqueous solvent. To learn the techniques of drying a dehydrated solution. Use the remaining aqueous layer for a second extraction.
This technique can be used to separate covalent molecules from ionic compounds in an aqueous solution or suspension. EnMohamad Fahrul Radzi Date of Submitted. The organic solvent can be any solvent that is immiscible with water.
It is a chemical process of separation that consist of the separation of a substance from the matrix. - To learn the techniques of separating toluene from water and other inorganic compounds by extraction. EXTRACTION AND DRYING OF AN AQUEOUS SOLUTION.
Extraction Unit Soxhlet Extractor Unit Drying of Aqueous Solution Fractionation Column Unit Organic Solution Particle Formation Unit. It is also held to learn the techniques of drying the extracted organic solution. The new technique is promising for achieving absolute sensitivity charging every analyte molecule and for performing spatially-resolved analysis of liquid.
Percentage yield of toluene recovered. If the drying agent remains undissolved after 15 minutes then discard the aqueous solution still in the 250 mL beaker down the drain. Nurul Nabila Binti Abu Bakar Class.
Stopper the funnel and shake as in an extraction. The mass spectra of intact molecular ions obtained from aqueous solutions of peptides and pro- teins are presented. On a microscale level it is easiest to pass the organic solution through a drying column.
Extraction and drying of an aqueous solution result. Old drying agent and add fresh drying agent to the filtered solution if it becomes wet looking or clumped. Extraction is a technique that commonly used to separate an organic compound from an aqueous.
Liquidliquid extraction LLE also known as solvent extraction and partitioning is a method to separate compounds or metal complexes based on their relative solubilities in two different immiscible liquids usually water polar and an organic solvent non-polar. Flask as the distillation flask. Combine the aqueous extracts and set them aside in a labeled flask.
Where ionic species are removed from a non-polar solvent by extraction into water. If the drying agent forms a clump at the bottom of the tube then more drying agent is needed. DISCUSSIONS Extraction and Drying of An Aqueous Solution is an experiment held to learn the techniques of separation of organic compounds such as toluene from water by extraction.
To learn the techniques of drying a dehydrated solution. 11ml 15ml 100 73. View CHM207-EXTRACTION AND DRYING OF AN AQUEOUS SOLUTIONdocx from CHM 207 at Universiti Teknologi Mara.
Doc Inorganic Chem Mariam Hanani Ismail Academia Edu
Separating Components Of A Mixture By Extraction Youtube
Esters And Esterification Chemistry Tutorial
4 6 Step By Step Procedures For Extractions Chemistry Libretexts
Extraction Apparatus An Overview Sciencedirect Topics
Lab 2 Docx Course Code Chm207 Experiment 3 Extraction And Drying Of An Aqueous Solution Name Intan Nurfarzana Binti Mohd Safini Student Id Course Hero
4 6 Step By Step Procedures For Extractions Chemistry Libretexts
Extraction And Drying Of An Aqueous Solution Name Organic Chemistry Uitm Studocu
Separating Components Of A Mixture By Extraction Youtube
4 8 Acid Base Extraction Chemistry Libretexts
Chm207 1c Docx Experiment 1c Extraction And Drying Of An Aqueous Solution Name Rawaida Binti Razali 2017257856 Group Members Muhammad Aidan Bin Course Hero
Separation Of An Unknown Mixture
4 6 Step By Step Procedures For Extractions Chemistry Libretexts
Experiment 1c Extraction And Drying Of An Aqueous Solution Docx Experiment 1c Extraction And Drying Of An Aqueous Solution Objectives 1 2 To Learn Course Hero
- shell out puncak alam
- kios jualan
- saiz frame tingkap
- penang bridge marathon 2016
- contoh lukisan realistik
- obat perut kembung bayi 1 tahun
- pantai hospital ayer keroh
- model dapur cantik rumah minimalis
- jenis konsep ruang
- kata kata mutiara ingin bertaubat
- the sugar book malaysia
- columbia asia petaling jaya
- pelaporan pbs tahun 6
- contoh abstrak untuk proposal
- bubur labu kuning santan
- No Keywords
- malaysia technology development corporation
- kata kangen dengan kekasih
- pagar kayu tahan cuaca
- sajak kanak-kanak
